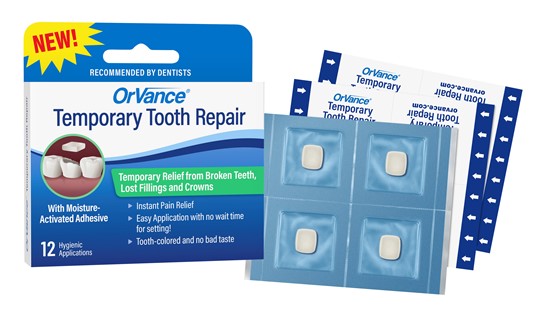
OrVance Announces Development of New Temporary Tooth Repair OTC Product with Strong Support from Dental Professionals

GRAND RAPIDS, MICHIGAN – November 1, 2021 – OrVance LLC, a developer of proprietary consumer oral health products, announced the launch of OrVance® Temporary Tooth Repair (TTR™) as a next generation product in the over $20 million At-Home Dental Cement retail segment. The company’s newest product is indicated to provide temporary relief from broken teeth, lost fillings and crowns until proper dental treatment and is expected to be on retail shelves in 2022.
The company also released key research findings from a survey of dentists on the At-Home Dental Cement retail segment and OrVance® TTR as a safe and effective alternative. Key findings include:
- 91% of dentists surveyed would prefer their patients use OrVance® TTR vs. one of the At-Home Dental Cement leading brands.
- 94% say hygienic (single-use) packaging is important for a product used for broken teeth, lost fillings and crowns (OrVance® is the first to offer hygienic packaging in the Temporary Tooth Repair segment and the Orthodontic Wax segment with its patented OrthoDots® CLEAR product).
- Only 43% of Dentists surveyed have recommended At-Home Dental Cement in the past while 94% say they would recommend OrVance® TTR to their patients if it were available at major drug store chains.
- The majority of dentists do not recommend their patients re-cement crowns at home. 83% would prefer their patients use OrVance® TTR only to provide temporary relief from dentinal hypersensitivity until a scheduled appointment for proper re-cementing in the practice.
Dental professionals can learn more about the new OrVance® TTR by watching
this quick instructional video.
According to Ron Schutt, OrVance’s CEO, “We’re excited about our expansion into the Temporary Tooth Repair self-care segment. Not only do we believe our TTR™ product is well positioned to become the number one brand but we estimate its ease of use, being the first temporary solution for broken teeth, along with higher recommendations from dental practices will expand this retail segment to over $45 million in the U.S.” Schutt Continued, “And as OTC products for dental emergencies are most commonly purchased in the brick-and-mortar retailers, our plan is to launch this product with an established CPG company in the oral care segment.”
Dr. Brian Tyler, Dentist and OrVance Advisory Board Member stated, “OrVance has again proven its ability to bring meaningful innovation to oral health with this TTR product. First by discovering the unmet need for temporary relief from broken teeth in its consumer research back in January of 2020. Then when all our dental practices were closed due to COVID-19, we learned that our patients in need of temporary relief used OrVance’s OrthoDots® CLEAR with very high satisfaction. So it’s now obvious that providing a hygienically packaged, tooth-colored, pliable material that sticks will be a winner with consumers and supported by the dental profession as a safe and effective remedy until permanent treatment.”
About OrVance
OrVance LLC is a developer of proprietary oral health products and is based in Grand Rapids, Michigan. It is over 90% owned by orthodontists, dentists and its managing partners. Learn more at orvance.com.
###
Contact:
Craig Clark
(616) 550-2736
craig@clarkcommunication.com


