MSU Student Research Reveals That Moms Need To Know About The Deficiencies Of Dental Wax
Advertising classes offer promotional campaigns for only orthodontic wax product that meets current quality and regulatory standards

(EAST LANSING, MI) – April 30, 2020 – Marketing majors from two Michigan State University advertising capstone classes recently completed a comprehensive project to recommend market expansion plans for OrthoDots® CLEAR, a Michigan-made product that is proven to be a superior alternative to commodity dental wax commonly dispensed by orthodontists. The findings from the 51 senior-level students, many of whom had undergone orthodontic treatment in the past, revealed that parents (namely, moms) should be more aware of the quality, safety, and regulatory deficiencies of conventional dental wax.
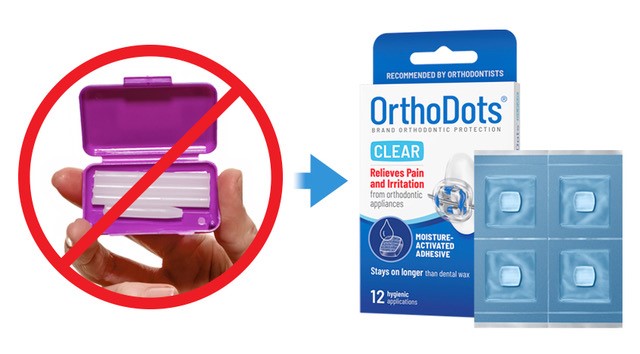
Developed and manufactured by Grand Rapids-based OrVance, OrthoDots® CLEAR not only out performs traditional dental wax, but it is also the first orthodontic wax to meet critical quality and regulatory standards such as hygienic packaging, tamper-evidence, disclosure of ingredients, and fully complies with Food and Drug Administration (FDA) labeling regulations related to product traceability.
The two “Integrated Campaigns” classes grouped in nine different teams, led by professor Louis Schiavone, recently reported its findings to OrVance.
According to Ron Schutt, CEO of OrVance, “Many of these students had personal experience with orthodontic treatment and the deficiencies with dental wax, so we were extremely impressed with the relevant insights and very professional recommendations they offered. I want to especially thank Lou Schiavone and his students who did an excellent job in helping us better focus our marketing and advocacy efforts. I’m inspired by the level of professionalism these Spartans brought to this project, especially considering that much of the semester was done remotely during this COVID-19 shutdown.”
The common themes that were consistently addressed throughout the nine breakout groups included:
- In addition to the superior performance and aesthetic features, OrVance should focus more on educating consumers on the hygienic benefits of OrthoDots® CLEAR versus conventional dental wax, which offers patients no hygienic packaging or safety seals.
- OrVance should shift its focus to educate moms, the primary stakeholders, who will ultimately demand compliance with current quality and safety standards within the orthodontic industry.
- In addition to heightening awareness for the need for hygienic packaging, especially in the current climate, moms will also appreciate the OrthoDots® CLEAR tamper-resistant packaging, the disclosure of ingredients, and the brand’s full compliance with FDA labeling regulations.
- In their campaigns, the teams consistently acknowledged OrthoDots® CLEAR as a substantial innovation to enhance the comfort and satisfaction of patients in clear aligner trays as well as braces.
The teams recommended OrVance highlight that OrthoDots® CLEAR is made in the United States and complies with FDA labeling regulations requiring the disclosure of the product’s origin, whereas a very high percentage of the noncompliant dental wax is being imported, most commonly from China.
“We enjoyed working with OrVance on this project,” said Schiavone. “Clearly, OrVance has brought to market a meaningful innovation to enhance orthodontic treatment and our students came up with some highly creative approaches that will accelerate acceptance, particularly among moms and children who are undergoing orthodontic treatment.”
OrVance LLC is a developer of proprietary oral care products and is based in Grand Rapids, Michigan. It is over 90% owned by orthodontists, dentists and its managing partners.


